NGS Solutions for Emerging Nipah Outbreaks
Combatting the Nipah Threat: Rapid Targeted NGS Solutions for Emerging Viral Outbreaks
By LexigenBio Scientific Team
As Nipah Virus (NiV) outbreaks continue to emerge across India and parts of Southeast Asia, the global health community is on high alert. Classified as a WHO priority pathogen, NiV is a zoonotic RNA virus with a staggering mortality rate of 50% to 75%. With its ability to cause severe respiratory distress and fatal encephalitis, the window for effective intervention and epidemiological containment is exceptionally narrow.
In the face of such a lethal threat, traditional diagnostic methods often fall short. LexigenBio is proud to offer the LeXso NiV Pathogen Detection Comprehensive Solution—an integrated, high-velocity NGS ecosystem designed for the precise identification and surveillance of Nipah Virus.
The Challenge: Detecting a Biosafety Level 4 (BSL-4) Pathogen
Nipah Virus (NiV-MY and NiV-BD strains) presents several diagnostic hurdles:
- High Lethality & Low Load: Early-stage patients may have low viral titers, requiring ultra-sensitive detection limits.
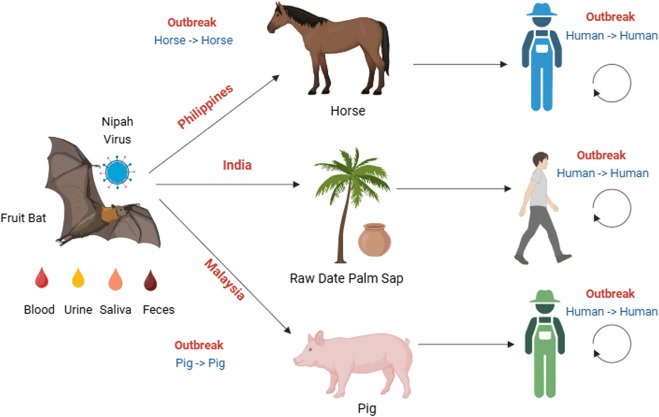
- Complex Transmission: With fruit bats as reservoirs and pigs as intermediate hosts, tracking the virus across “One Health” interfaces requires robust sample compatibility (from environmental to clinical).
- Urgency: In an outbreak scenario, the traditional 24-48 hour NGS turnaround is too slow for containment.
The LexigenBio Response: The LeXso NiV Ecosystem
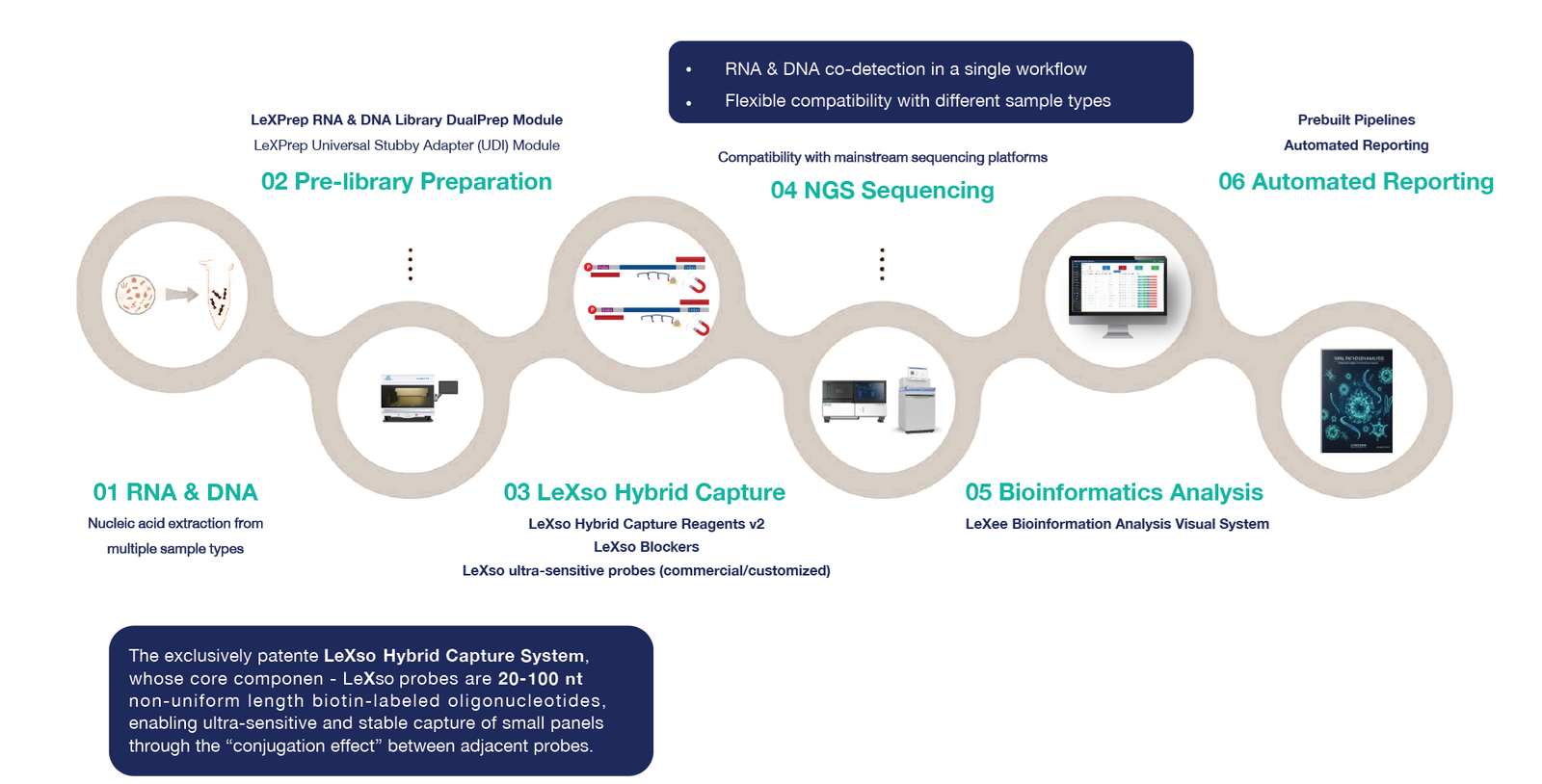
Our solution leverages the patented LeXso Hybrid Capture System to provide a “Sample-to-Report” workflow that is faster, more sensitive, and more comprehensive than standard mNGS.
1. Synchronous RNA & DNA Co-Preparation
Utilizing the LeXPrep RNA & DNA Library DualPrep Module, researchers can process mixed pathogenic samples in a single tube. Since NiV is a single-stranded RNA virus (-ssRNA), this module ensures that the viral transcript is captured with high fidelity alongside any potential DNA-based co-infections.
- Speed: 3–3.5 hours for co-library preparation.
- Stability: High conversion rates even with low-quality or fragmented samples.
2. Ultra-Sensitive LeXso NiV Panel v1.0
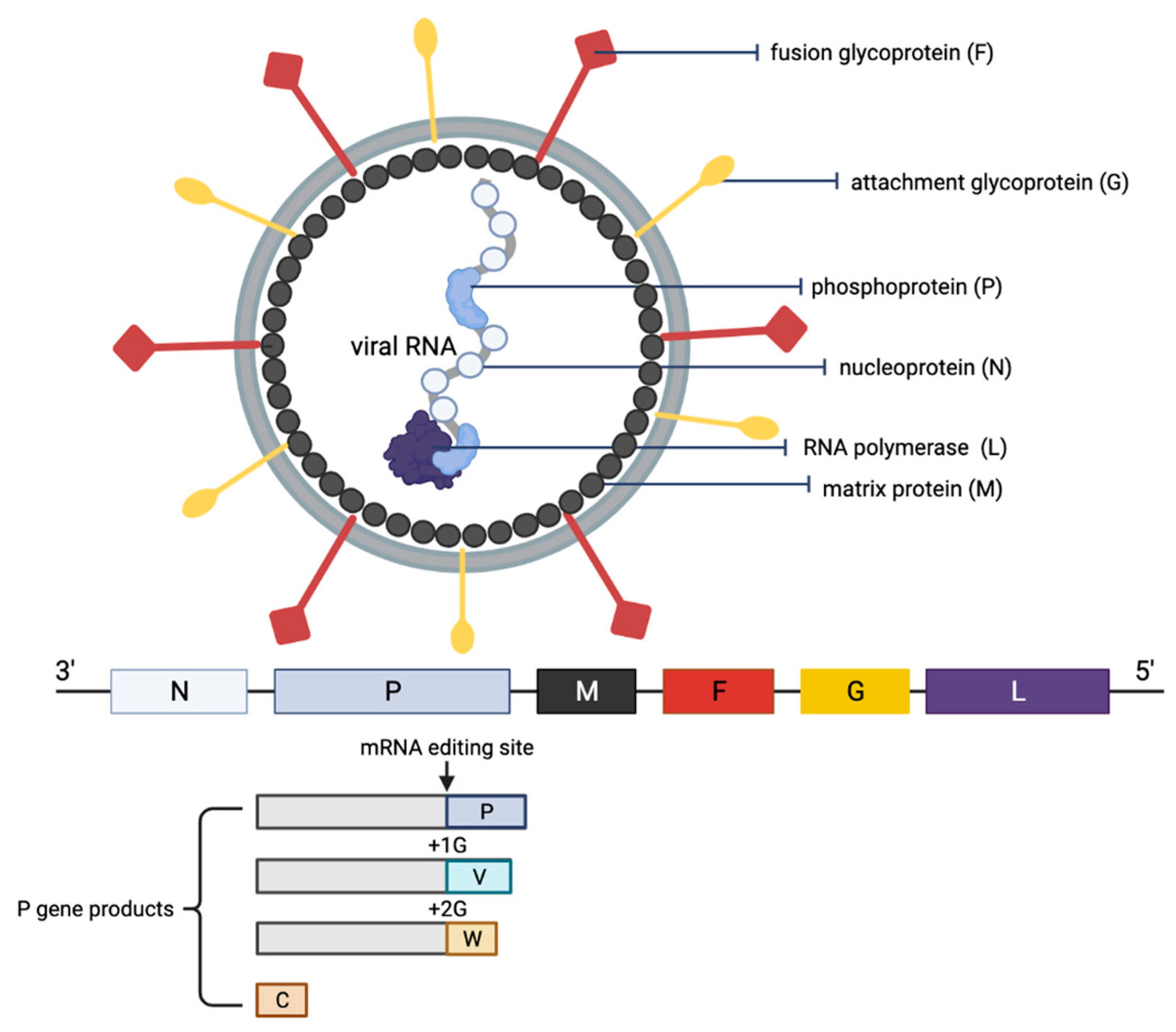
Our specialized panel targets the complete ~18.2 kb genome of both the Malaysia (NiV-MY) and Bangladesh (NiV-BD) strains.
- The Conjugation Effect: Our 20-100 nt non-uniform probes utilize the “conjugation effect” between adjacent probes to stabilize capture, enabling the detection of NiV sequences at ultra-low frequencies.
- Broad Coverage: Probes are strategically tiled across the N, P, M, F, G, and L coding sequences to ensure accurate typing and variant tracking.
3. Breaking the 16-Hour Barrier
While traditional hybridization takes 16–24 hours, the LeXso Hybrid Capture Reagents complete the targeted enrichment in as little as 1–2 hours. This allows laboratories to move from raw sample to actionable sequencing data within a single day.
Workflow: Sample to Insight in Under 12 Hours
- Extraction: Nucleic acid recovery from clinical or environmental samples.
- DualPrep Library Construction: Synchronous RNA/DNA preparation using LeXPrep.
- LeXso Targeted Capture: Rapid enrichment of NiV genomic markers using the LeXso NiV Panel.
- Sequencing: Compatible with both Illumina and MGI platforms.
- Automated Analysis: The LeXee Bioinformatics System provides automated reporting, identifying the specific genotype and identifying any novel mutations in the F or G proteins involved in host-cell entry.
Scalability via LeXBot Automation
During an active outbreak, manual pipetting is a bottleneck and a safety risk. The LeXBot Automation Series provides a “walk-away” solution for the entire NiV detection workflow, ensuring high-throughput reproducibility and minimizing operator exposure to potentially infectious materials.
Conclusion: Precision Surveillance for Global Health Security
The LexigenBio LeXso NiV Comprehensive Solution is more than just a diagnostic kit; it is a critical tool for global health security. By providing ultra-sensitive, same-day genomic evidence, we empower public health authorities to track viral evolution, map transmission routes, and ultimately save lives.
Is your laboratory equipped for the next outbreak? [Contact support@lexigenbio.com]
Ordering Information:
- LX02411: LeXPrep RNA & DNA Library DualPrep Module
- LX-NiV: LeXso NiV Panel v1.0
- LX15201: LeXso Hybrid Capture Reagents v2
Visit www.lexigenbio.com for more information.
Reference:
https://encyclopedia.pub/entry/24264
Asokan S, Luke MS, Atiyah HM, Noori SS, Atiyah MM, Makeshkumar V, Verma G, Jagadeesan A, Beniwal N, Vijayan S, Rajeswary D. Nipah virus as a pandemic threat: Current knowledge, diagnostic gaps, and future research priorities. Diagn Microbiol Infect Dis. 2026 Feb;114(2):117141.
Branda F, Ceccarelli G, Giovanetti M, Albanese M, Binetti E, Ciccozzi M, Scarpa F. Nipah Virus: A Zoonotic Threat Re-Emerging in the Wake of Global Public Health Challenges. Microorganisms. 2025 Jan 9;13(1):124.