Mastering Large Fragment Deletion Analysis in CGT
The CRISPR Safety Elephant in the Room: Mastering Large Fragment Deletion Analysis in CGT
By LexigenBio Scientific Team
Cell and Gene Therapy (CGT) is currently one of the most dynamic frontiers in medicine. From curing rare genetic disorders to engineering “living drugs” for cancer, the potential of CRISPR-Cas9 is undeniable. However, as the field matures and more therapies move toward clinical trials, the regulatory spotlight has intensified on one specific, often overlooked risk: Unintended Large Fragment Deletions.
While small indels are the intended outcome of many CRISPR edits, recent studies have shown that double-strand breaks can frequently lead to massive structural variations (SVs)—sometimes spanning tens of kilobases—that standard analytical methods simply cannot see.
The Regulatory Shift: FDA 2024 and Genomic Integrity
In early 2024, the FDA issued updated guidance for human genome editing products, placing a heavy emphasis on Genomic Integrity. Regulatory bodies now expect developers to provide a comprehensive “safety map” of their edited cells, including:
- Off-target analysis with high-sensitivity biochemical or cellular assays.
- Chromosomal abnormalities and large structural rearrangements.
- Clonal expansion risk assessment.
For CGT developers, the challenge is clear: traditional PCR and short-read NGS are no longer enough to prove safety.
The Blind Spot: Why Standard Methods Fail
Why are large deletions so hard to detect?
- PCR Bias: Standard PCR primers often “bridge” over large deletions or fail to amplify across massive gaps, leading to a total loss of signal for the very variants you need to find.
- Short-Read Limitations: Standard NGS excels at finding SNPs but struggles to align reads across complex breakpoints, often resulting in “shadow” regions where structural variations remain hidden.
- Low Allelic Fractions: In a pool of edited cells, a dangerous deletion might exist in only 0.1% of the population—too low for traditional depth-based tools to identify with confidence.
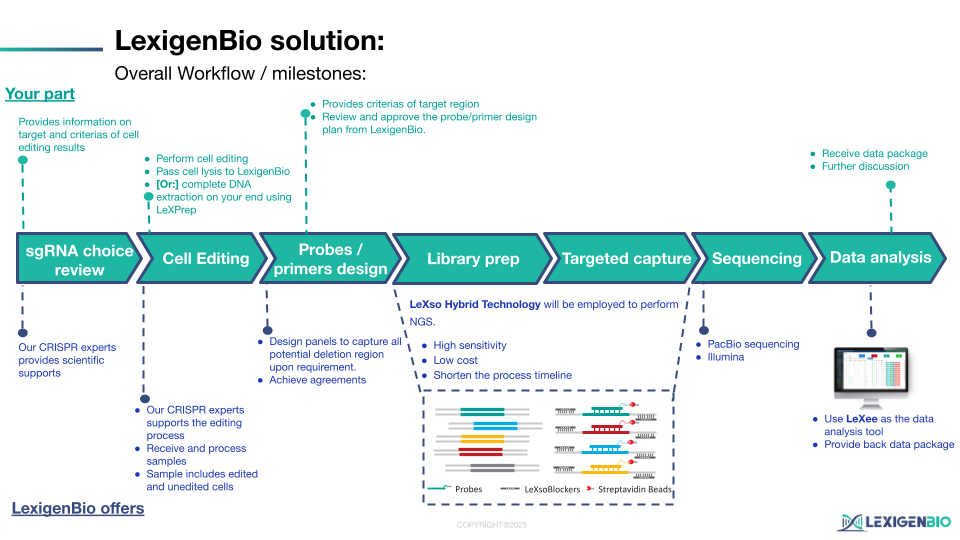
The LexigenBio Solution: High-Resolution SV Capture
LexigenBio has developed a specialized End-to-End Solution for CRISPR Large Deletion Analysis that combines high-fidelity probe capture with our patented LeXso kinetics.
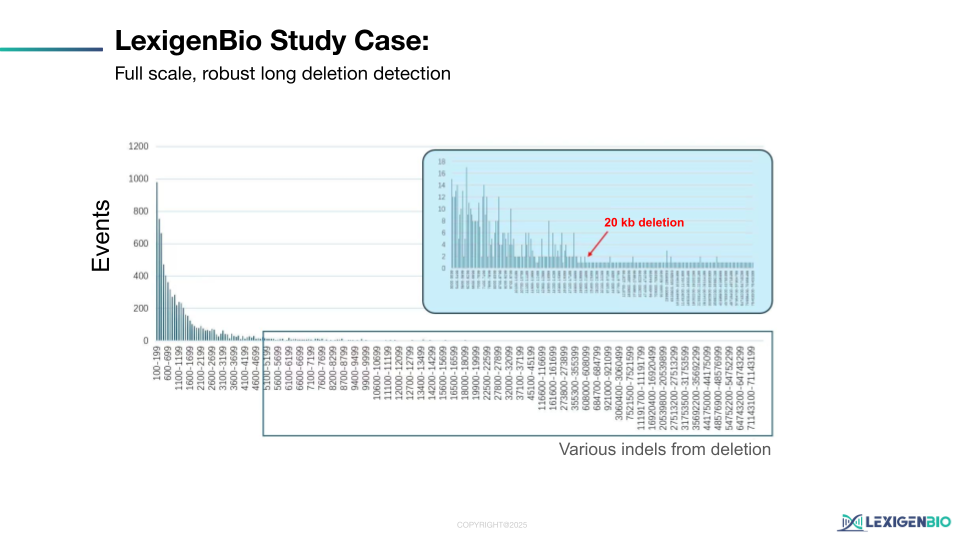
1. Panoramic Coverage (+/- 60kb)
Instead of relying on a single set of primers, our solution utilizes over 1,000 high-density probes tiled across a 120kb genomic window centered on the edit site. This ensures that even if a 20kb deletion occurs, the surrounding probes will capture the flanking sequences and precisely define the breakpoints.
2. The LeXso Speed Advantage
In the fast-paced world of CGT development, waiting 24 hours for capture results is a bottleneck. Our LeXso Hybrid Technology slashes hybridization time to just 1 hour. You can move from library to high-depth sequencing in a single afternoon.
3. High Sensitivity for Rare Repair Outcomes
Our system is optimized to detect structural variations at ultra-low allelic fractions. By maintaining high library complexity and using specialized UMI-based error correction, we can identify repair “scars” and large deletions with a sensitivity that meets and exceeds FDA expectations.
From Breakpoints to Bioinformatic Clarity
The final piece of the puzzle is our LeXee Visual Bioinformatics System. Raw reads are automatically processed through specialized structural variation pipelines that provide:
- Exact Breakpoint Mapping: Precise coordinates of where the genome was rejoined.
- Frequency Quantification: Accurate measurement of how many cells in your population carry the deletion.
- Visual CNV Plots: Intuitive graphs that make genomic integrity easy to present to stakeholders and regulators.
Conclusion: Safety is the Foundation of Innovation
As CRISPR therapies become more ambitious, the tools used to validate them must keep pace. LexigenBio is committed to providing the high-resolution evidence needed to bring life-changing therapies safely to market. By resolving the “SV puzzle,” we help you turn regulatory hurdles into a competitive advantage.
Is your CRISPR safety strategy ready for the clinic? [Request a Technical Solution Consultation]
Ordering Information Highlights:
- LX15201: LeXso Hybrid Capture Reagents (High-Speed Capture)
- LX02601: LeXPrep EZ DNA Library Preparation (Optimized for fragmented/low-input)
- Custom: Bespoke CRISPR Deletion Panels (+/- 60kb Targeted Design)
For more information, visit www.lexigenbio.com